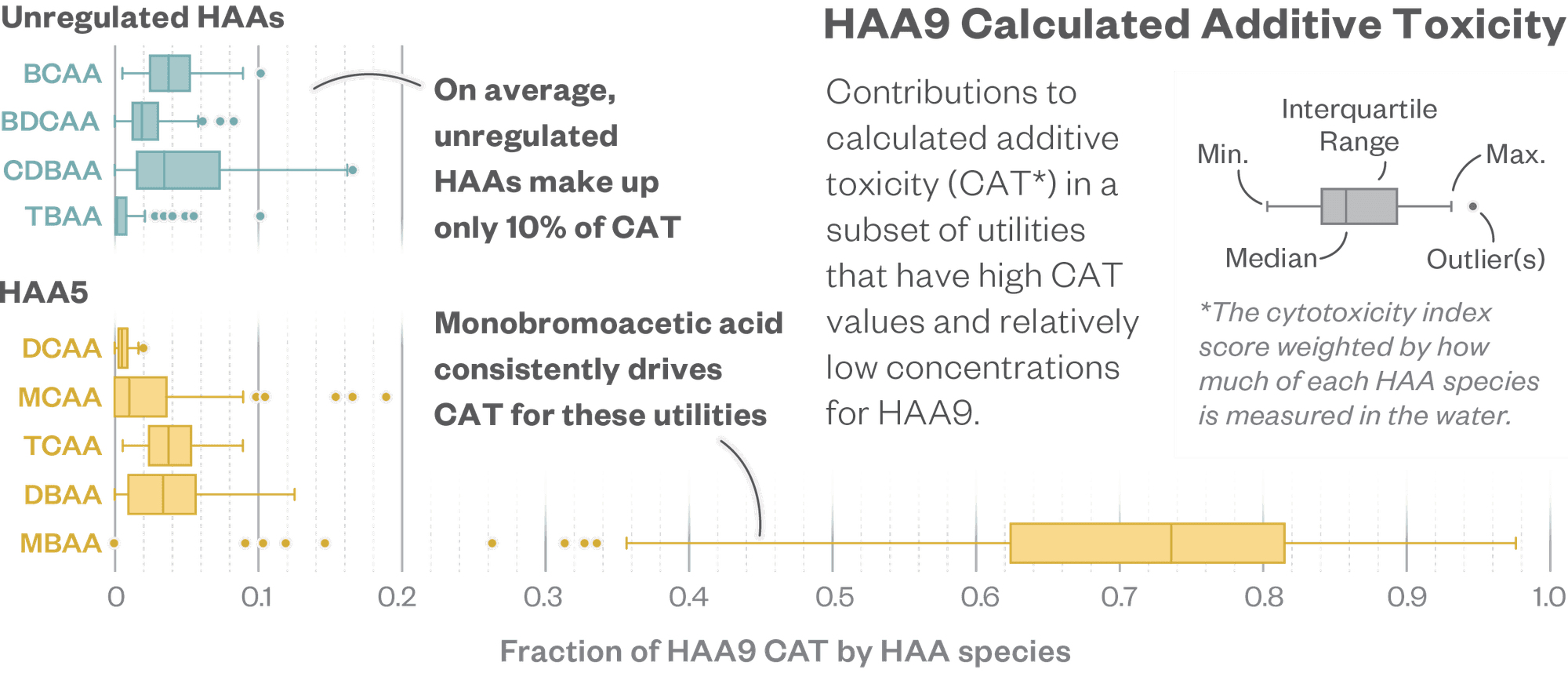Regulating These Chemicals is Unlikely to Achieve a Meaningful Benefit to Public Health
A Hazen-led project found that regulating more haloacetic acids, a kind of disinfection byproduct, probably won’t reduce people’s exposure to the most toxic ones.
At a Glance
- The U.S. Environmental Protection Agency is considering expanding disinfection byproduct (DBP) regulations for drinking water to include four more haloacetic acids (HAAs, a type of DBP). But a research project including Hazen, the consulting firm Intertox, and the University of Massachusetts Amherst—and funded by the Water Research Foundation—found that the expansion is unlikely to reduce people’s exposure to the most toxic HAAs.
- The team reached those conclusions through robust research, including an analysis of detailed water sample data from thousands of utilities and a comprehensive toxicity evaluation of 69 DBPs.
- Monobromoacetic acid (MBAA)—one of five HAAs that are already regulated—is the most cytotoxic HAA. (Cytotoxicity means the ability to damage living cells.) Researchers found that MBAA is the biggest driver of HAA cytotoxicity for utilities currently in compliance with DBP regulations.
- MBAA is also one of the most difficult HAAs to measure accurately. Due to its importance for HAA toxicity, the team recommends the development of better methods for measuring MBAA.
- The four additional HAAs under consideration don’t seem to have much impact on the overall toxicity of HAAs in drinking water.
Related Solutions:
"Going into this project, we thought it might make sense to expand the HAA5 regulation to HAA9. Those four additional HAAs are not hard to measure now, and based on their chemical makeup, we thought they could be fairly toxic. When we ran the numbers, that just wasn’t the case." Billy Raseman, PhD, who led Hazen’s leg of the DBP toxicity study
The EPA is considering regulating more disinfection byproducts (DBPs).
In the early 1900s, U.S. cities began using chlorine to kill harmful pathogens in drinking water. Deaths from infectious diseases plunged as the practice spread. But by the ‘70s, researchers had discovered that the disinfection process was unintentionally creating a different set of chemical compounds. Disinfection byproducts (DBPs), as they’re now called, are formed when chlorine, chloramine, and other disinfectants react with naturally occurring organic material in the water.
Research has since found associations between high levels of DBPs in drinking water and a number of health problems, including bladder cancer. The U.S. Environmental Protection Agency (EPA), meanwhile, has imposed collective concentration limits in drinking water for two groups of DBPs: 80 micrograms per liter (µg/L) for total trihalomethanes (TTHM) and 60 µg/L for five haloacetic acids (HAA5). The agency is considering adding four more HAAs to the mix, for a total of nine regulated HAAs (HAA9).
But scientific knowledge of DBPs—including their potential health impacts—is playing catch-up with the regulations.
The links between DBPs and negative health impacts are still fuzzy, so it’s unclear which chemicals might be most problematic. So far, more than 600 DBPs have been discovered. The EPA started with limits for TTHM and HAA5 in part because they were the only groups the agency could confidently measure when those laws were enacted.
The agency can now measure the additional four HAAs that would be included in HAA9. On the one hand, expanding the regulation seems prudent: There’s growing evidence that brominated DBPs, a subset of the chemicals that includes those four HAAs, are particularly toxic. On the other hand, there is no clear evidence that their toxicity impacts human health.
There’s also growing concern among researchers that the most toxic DBPs might fall entirely outside of the HAA and THM families, and that those other chemicals don’t always show up alongside HAAs and THMs. If that’s true, it would mean THMs and HAAs can’t be used to determine whether or not those other chemicals are present.
Leading DBP experts from Hazen, the consulting firm Intertox, and the University of Massachusetts Amherst partnered to investigate the various implications of the potential HAA9 rule. Here’s what the team has found.

Some HAAs appear to be far more toxic than others, and the most toxic one is already regulated.
As mentioned, the EPA currently requires maximum concentration limits for two groups of DBPs: 80 µg/L for total THMs (TTHM) and 60 µg/L for five HAAs (HAA5). The regulation gives equal weight to each individual chemical in each group. For example, for HAA5, a utility can have up to 60 µg/L of just one HAA from that group, or up to 60 µg/L of any combination. As long as those five HAAs total no more than 60 ug/L, the utility is within compliance.
But what if some HAAs are more toxic than others? What’s the best way to even determine which HAAs, and which DBPs in general, are most toxic? To answer those questions, researchers from Intertox completed a comprehensive review of DBP research that included 69 individual compounds across 10 groups.
To compare different chemicals, apples to apples, you need consistent data. When it comes to chemical exposure, the best data points are the ones that measure tangible impacts on human health—cancer, for instance. But it can take decades for researchers to confirm whether a chemical can increase someone’s cancer risk. And that’s just one health outcome for one chemical. There are more than 600 known DBPs, and the data on how they impact people are still scant. Even data on the impacts of DBPs in live animals proved too scarce and varied to use for a robust comparison.
Ultimately, the team used petri dish studies that measured DBPs’ cytotoxicity—their ability to damage individual mammal cells—because that was the most comprehensive data available. They came up with an estimated “weight” for each DBP: a measure of how toxic it seemed to be based on how much cell damage it caused. The weights say little about what these chemicals could mean for humans and animals, but they’re a useful way to screen and rank DBPs based on their toxicity to cells.
The team found that toxicity varies widely across DBPs, including within the HAA family, as the graphic above illustrates. Based on their cytotoxicity index, the most toxic HAA is one called monobromoacetic acid (MBAA). It’s part of the HAA5 group that’s already regulated and appears to be tens to hundreds of times more toxic than other HAAs.
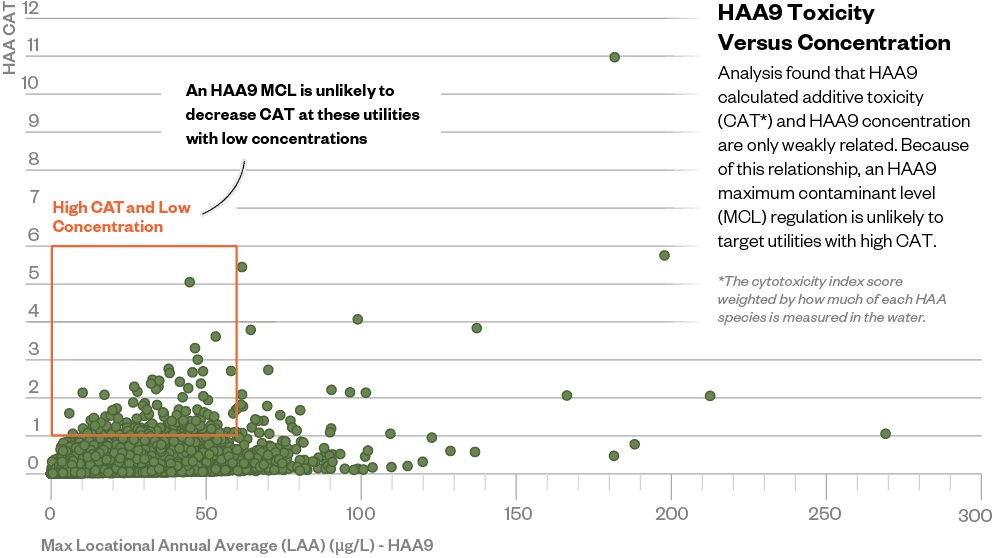
Expanding HAA5 to HAA9 would likely target the wrong utilities for the wrong reasons.
Because MBAA appears much more toxic than other HAAs, even low concentrations of it can drive up toxicity levels in water samples. That’s what Hazen’s engineers found when they looked at EPA data.
To inform its decision-making—and as part of the Fourth Unregulated Contaminant Monitoring Rule (UCMR 4)—the EPA required thousands of utilities across the country to sample their water for the HAAs that would be included in the HAA9 group, along with a bunch of other unregulated chemicals under consideration. Hazen obtained detailed records of the UCMR 4 data through a Freedom of Information Act request.
For each water sample, the team multiplied the concentration of each HAA by its toxicity value from the index, then added the results to get a value called calculated additive toxicity. Think of it as a “toxicity weight” for each water sample: the heavier the weight, the higher the toxicity of the combined HAAs in that sample.
The team found that a utility could have low total HAA9 concentrations but high toxicity weight if MBAA, the most toxic HAA, was present in the water (see the above graphic). What’s more, since MBAA is already regulated, most of the utilities with those high toxicity values are already accounted for within the EPA’s HAA5 regulation. An expanded rule likely wouldn’t affect them at all.
On the flip side, utilities with high HAA9 concentrations often had low toxicity weights, meaning they had a lot of the relatively less toxic HAAs. Requiring them to lower their concentrations wouldn’t provide a significant benefit, because it wouldn’t lower people’s exposure to the more toxic compounds.
These findings are initial. A more precise detection method for MBAA—and more robust data for that chemical—would strengthen them.
The team’s conclusions are based on the best available data and research. But there are two caveats to the data on MBAA. First, there still isn’t a highly accurate way to measure it. That means the EPA-approved detection method used to measure MBAA for the UCMR 4 data set could be somewhat over- or underestimating MBAA’s presence.
And second, that detection method only registered levels of MBAA as low as 0.2 µg/L. In most samples, MBAA wasn’t detected, which means we don’t know what the true concentration was. Many of the samples that did have measurable levels of MBAA had levels just above that 0.2 µg/L detection limit.
All of this evidence suggests that regulating the four additional HAAs EPA is considering wouldn’t significantly reduce people’s exposure to the most toxic HAAs. And it could create an unnecessary burden for utilities with high HAA9 concentrations.
The University of Massachusetts Amherst is now using bench-scale testing to expand these insights beyond the questions specific to HAA9. The tests will provide partner utilities with more detailed understanding about how different regulatory decisions, treatment methods, source water characteristics, and DBPs could impact their operations—and how they can target the most toxic DBPs, regulated and unregulated, to maximize public health protection.